Bridging the Gap: Biomedical Innovation for a Healthier Tomorrow
June 26, 2024
Picture a state-of-the-art facility, meticulously designed to transform groundbreaking scientific discoveries into life-saving medicines. This facility operates under strict guidelines known as Good Manufacturing Practices (GMP), ensuring every step of the manufacturing process meets rigorous quality standards.
Inside, teams of experts meticulously cultivate and process biological materials, harnessing the power of living organisms to create cutting-edge therapies. Their products could be anything from vaccines that protect against diseases to novel cancer therapies. Every aspect of the facility is geared toward precision and safety, with advanced technology and rigorous protocols to guarantee the purity, potency, and consistency of the final products. From the sterile cleanrooms where delicate processes unfold to the sophisticated quality control measures that scrutinize each batch, this facility represents the pinnacle of scientific innovation and medical progress.
In essence, it's a hub of hope and progress, where science and technology converge to create a healthier, brighter future for all.
Dalhousie and its partners are creating a biomanufacturing facility to bring this vision to reality. Their goal is to conduct preclinical studies and proof-of-concept studies that will ultimately translate medical discoveries into health solutions, bridging the gap between groundbreaking research and meaningful clinical applications.
The Atlantic Region, like the rest of Canada, is grappling with significant healthcare challenges including an aging population, rising rates of cancer and chronic inflammatory conditions, and increased vulnerability to infectious diseases. In response to these pressing issues, Dalhousie University's Faculty of Medicine, in collaboration with Nova Scotia Health, the IWK Health Centre, the Centre for Canadian Vaccinology, and Life Sciences Nova Scotia—have leased a production facility in Dartmouth that will allow them to make their vision a reality.
“We have world-leading health research right here in Atlantic Canada, yet without the systems, facilities, or skilled workforce, this research stalls in the lab and never reaches the people who need it.”
Dr. David Anderson, Dean of Medicine, Dalhousie University
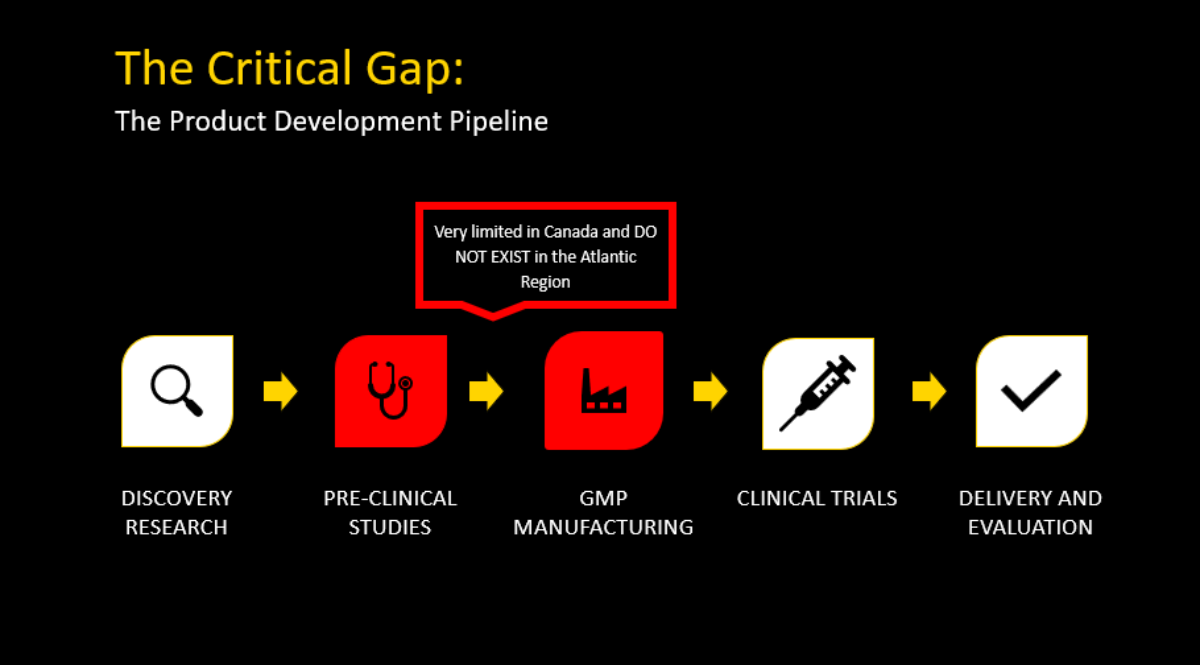
The Critical Gap
Despite a solid track record of discoveries and innovations from our top researchers, previously, home-grown medical breakthroughs often had to be tested and developed outside the province in order to develop lifesaving drugs or therapies. .
Many discoveries failed to progress beyond the research phase due to a lack of infrastructure, support, and the systems necessary to manufacture and test them. The COVID-19 pandemic highlighted this gap, emphasizing the urgent need for pre-clinical studies and small-scale manufacturing facilities across Canada. This new facility is our solution to propelling medical innovations from discovery to clinical application.
What the pandemic taught us was that by prioritizing collaboration, industry partnerships, specialized facilities, and skilled personnel, we can shorten the traditional 15-year innovation pipeline significantly. This new biomanufacturing facility seizes this opportunity to accelerate the translation of research into real-world health solutions.
The Solution: A Collaborative Biomanufacturing Facility
The new facility will be a catalyst for Atlantic-born medical breakthroughs to transform healthcare outcomes regionally, nationally, and globally. Researchers will be able to use this space to conduct pre-clinical studies to establish the safety and effectiveness of new vaccines and pharmaceuticals and manufacture small batches for proof-of-concept and product testing. This testing, required to attract industry partners, will reduce our reliance on external facilities and organizations.
Our facility will also train highly skilled technicians and build a highly qualified workforce to support the world-leading researchers at Dalhousie.
Bridging the Gap
The biomanufacturing facility is designed to be the ecosystem Atlantic Canada needs for medical innovation. It addresses the challenges researchers face in navigating the complex journey from discovery research to clinical trials. By providing a clear research pathway and providing resources they don’t currently have access to, the facility empowers researchers to focus on what they do best—advancing medical discoveries that can change lives.
- Critical Infrastructure: Specialized Spaces and Equipment
To enable world-class research and discovery, the facility will invest in purpose-built, fully equipped spaces. This includes a lab and small-scale manufacturing facility, crucial for conducting the pre-clinical studies necessary to attract the funding for larger-scale clinical trials.
- Expertise and Systems: The Brain and Backbone of the Facility
Recognizing the diverse skillsets required for advancing medical discoveries, the facility will assemble leadership teams and skilled support personnel. They will conduct research, manage projects, and work closely with partners at other emerging vaccine and therapeutics teams across Canada. The facility’s teams will guide work through the research pathway, ensuring critical resources are available for successful translation from idea to finished product. The new facility will l streamline the development process for new drugs, vaccines, and other therapeutic products.
- Training Programs: Growing a Highly Skilled, Industry-Ready Workforce
Collaborating with industry and corporate partners, the biomanufacturing facility will establish programs to train the highly skilled personnel the biomedical technology and life sciences industries require. These programs will focus on moving bioscience research and innovation into clinical practice. By attracting top-tier talent, the facility will increase skilled workers in these fields nationally and support the growing bioeconomy in Atlantic Canada.
Innovation is Good. Activation is Better
Dalhousie's I3V team—Infection, Immunity, Inflammation and Vaccinology—has a history of key discovery research. The biomanufacturing facility will be the catalyst to translate their findings into revolutionary, life-changing solutions. With specialized spaces, expertise, and support systems, this new space will guide promising discoveries from this group of researchers and others, through the biomedical research pathway, ultimately delivering critical health solutions.
The Timing is Right, the Time is Now
With Dalhousie's established expertise and a favourable environment of unprecedented federal financial investments, the time is ripe for this innovative opportunity.
The federal government has recognized Canada’s urgent need to expand its domestic biomanufacturing capabilities and strengthen the Life Sciences sector, committing $2.2-billion in Budget 2021 to a Biomanufacturing and Life Sciences Strategy. This national investment will establish four biomanufacturing hubs across the country. Dalhousie is poised to be a key partner in three of these hubs, and our new Biomanufacturing facility will be critical to those partnerships. Philanthropic donations will be critical to fully realize the potential the new facility has to bring our research discoveries to the bedside.
The collaborative biomanufacturing facility represents a beacon of hope for Atlantic Canada and beyond, addressing critical gaps in healthcare innovation. By providing the necessary infrastructure, expertise, and support, we aim to transform medical discoveries into solutions that improve health outcomes and save lives.
As Dalhousie's I3V team takes the lead, the facility will mobilize our best minds and resources to tackle the pressing healthcare challenges of our time.
